
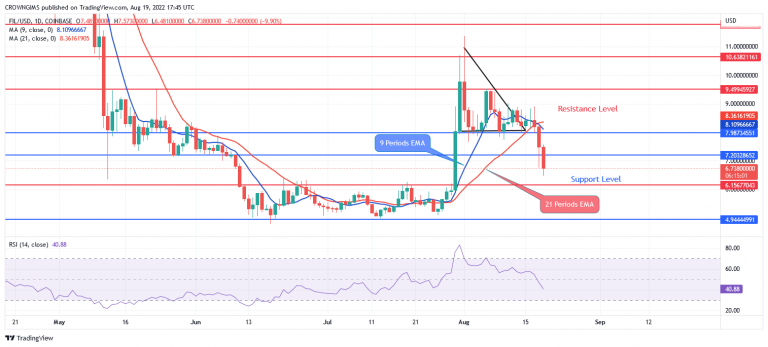
We look forward to continuing to collaborate with our partners at CSL Vifor and with the EMA throughout the review process.” “The acceptance of the CMA application marks an important next step on our pathway to expanding access to sparsentan as the first non-immunosuppressive treatment option for IgA nephropathy in Europe, if approved. chief medical officer of Travere Therapeutics. FDA’s acceptance and granting of priority review of the NDA for sparsentan for IgA nephropathy in the U.S., we continue to make great progress towards our goal of enabling sparsentan to become a new treatment standard for rare kidney disorders, if approved,” said Jula Inrig, M.D. “We look forward to working closely with our partner, Travere, through the EMA review process with the aim to bring this innovative treatment option to patients living with IgAN in Europe.” “The acceptance of the EU regulatory application for sparsentan marks a major milestone towards our goal of bringing this first-in-class therapy to the patients suffering from IgAN, for which there are currently no approved non-immunosuppresive therapies,” commented Klaus Henning Jensen, Chief Medical Officer of CSL Vifor.

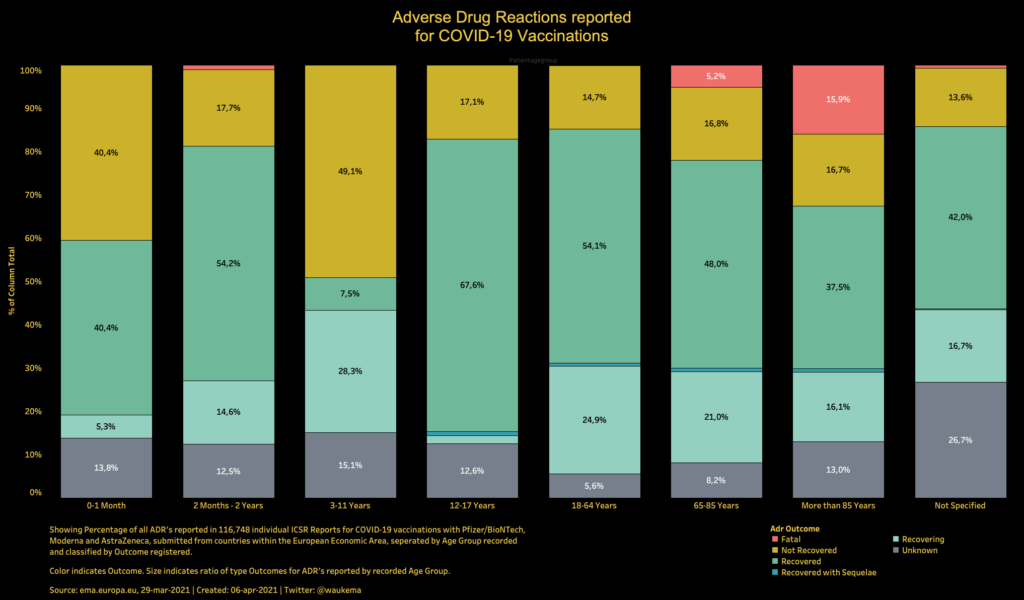
The EMA will review the application under the centralized marketing authorization procedure and a review decision on a potential approval is expected in the second half of 2023. (NASDAQ: TVTX) today announced that the EMA has accepted for review the Conditional Marketing Authorization (CMA) application for sparsentan for the treatment of IgAN, a rare kidney disorder and a leading cause of end-stage kidney disease (ESKD). If approved, sparsentan will be a first-in-class treatment to address the significant unmet medical need in IgA nephropathy (IgAN) in EuropeĬSL Vifor and Travere Therapeutics, Inc. Gallen, Switzerland & San Diego, United States:Ī review decision by the European Medicines Agency (EMA) is expected in the second half of 2023


 0 kommentar(er)
0 kommentar(er)
